Our commitment to innovative research and clinical advancements leads to groundbreaking treatments.
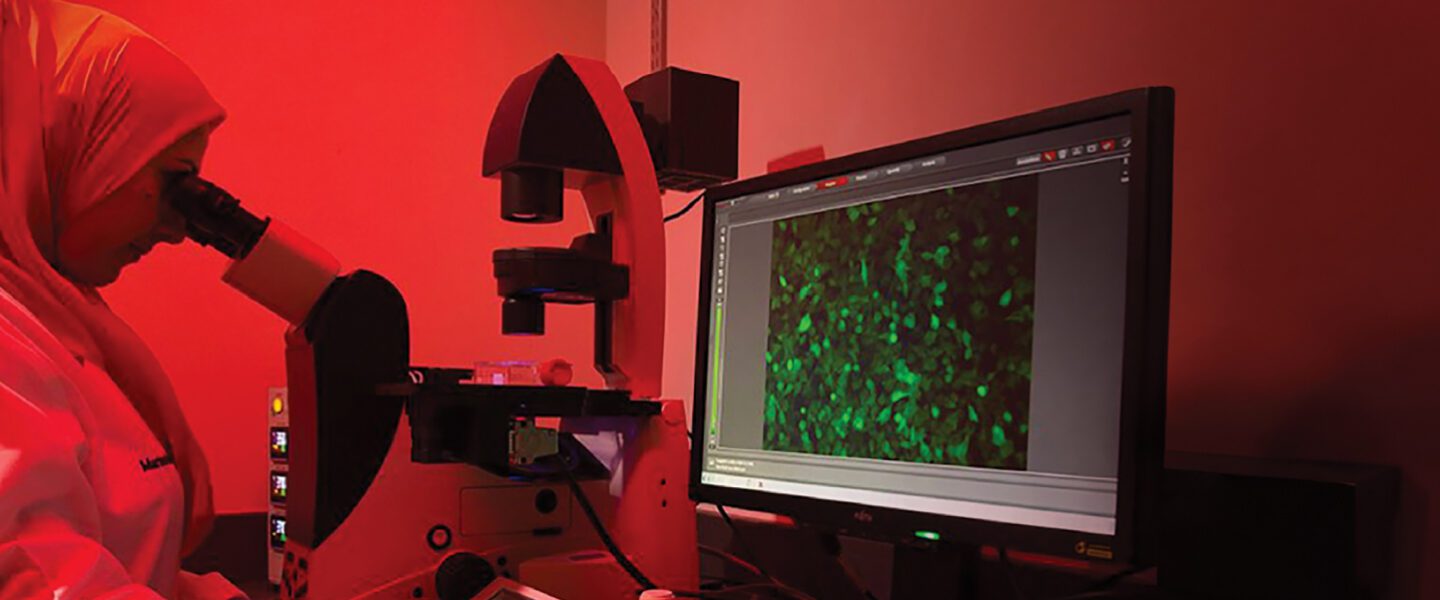
In healthcare, innovation is essential to the advancement of patient care. That’s especially true at HCA Healthcare, where innovation is a foundational value and continues to drive cancer research and treatment. HCA Healthcare’s Sarah Cannon Transplant and Cellular Therapy Network (SCTCTN) comprises nine programs in six U.S. locations and three locations in the United Kingdom. The network serves patients with complex blood cancers such as leukemia and those receiving blood and marrow transplant, cellular therapy, or gene therapy for blood diseases. In 2023, the network cared for more than 1,000 acute leukemia patients and 1,600 transplant and cellular therapy recipients. HCA Healthcare’s scale makes the enterprise the largest provider of complex blood cancer care in the U.S. But what truly sets us apart is the comprehensive network approach to patient care.
“Our physicians and teams work together to standardize patient care pathways, treatment regimens, quality plans and processes so that every patient receives the same high-quality care at all of our programs,” says Navneet Majhail, MD, MS, FASTCT, a board-certified hematologist and the physician-in-chief of blood cancers at SCTCTN, specializing in blood and marrow transplant and cellular therapy.
Our network approach enables us to integrate cutting-edge research for blood cancer patients. The partnership with the Sarah Cannon Research Institute provides access to collaborations ranging from first-in-human research studies to clinical trials that lead to Food and Drug Administration-approved blood cancer drugs.
Innovative cancer therapy
A shining example of the pioneering work underway at SCTCTN is chimeric antigen receptor T cell therapy, also known as CAR T-cell therapy.
“It is a transformational therapy that can potentially cure some patients with advanced cancers who previously had no good treatment options available,” says Dr. Majhail.
The FDA has approved CAR T-cell therapies for patients with advanced acute lymphoblastic leukemia, lymphoma and multiple myeloma. However, since T cells can be engineered to target any cancer antigen, significant research is being done to evaluate treatment for other blood cancers and in patients with solid tumors, such as those with GI cancers, brain tumors and lung cancer.
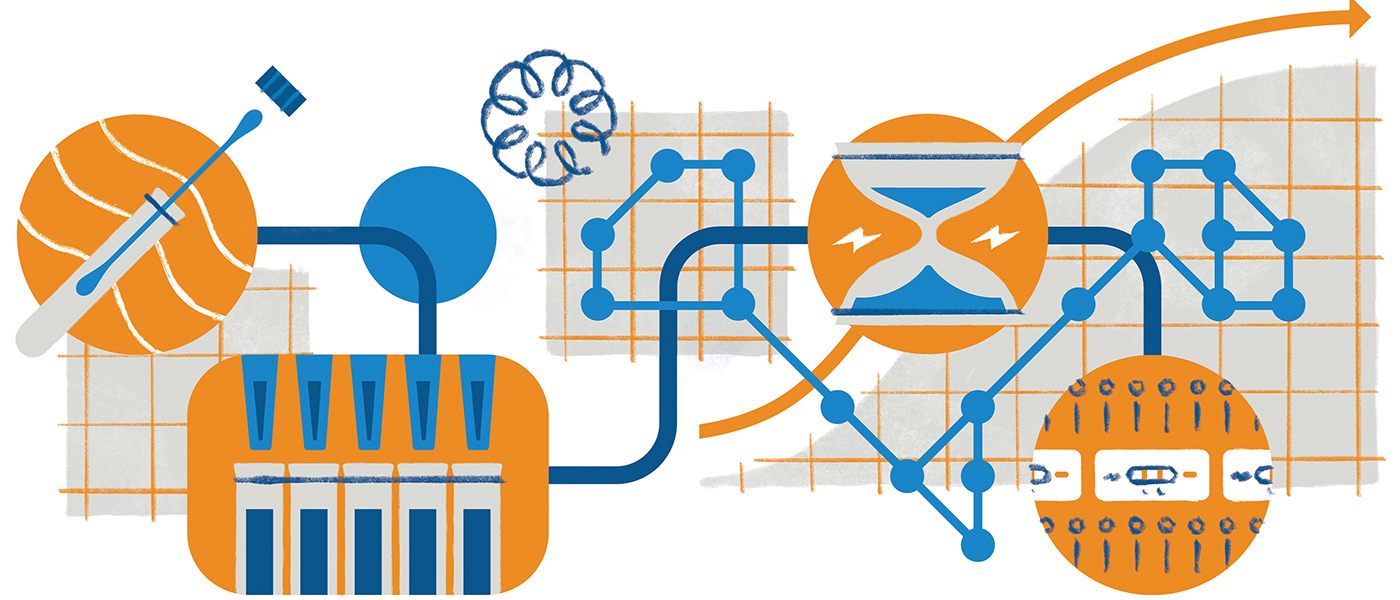
During the CAR T-cell treatment process, T cells are drawn from a patient’s blood and genetically engineered to recognize the patient’s cancer cells when reinfused. T cells are effective at killing cancer cells, but under normal circumstances they need the help of other immune cells to work efficiently.
“With the technology we have available now, these cells can be taken from the body and their DNA modified such that they can start identifying cancer proteins and antigens directly,” says Dr. Majhail.
Patients first undergo testing to ensure that they qualify for the CAR T-cell procedure. The patient’s T cells are then collected by an apheresis machine that filters out lymphocyte cells, which are sent off for manufacturing.
“Once ready, they are shipped back to us,” says Dr. Majhail. “Patients then receive chemotherapy to suppress their immune system, and the CAR T-cells are subsequently given as intravenous infusions. Patients are observed very closely for four to six weeks after the infusion to monitor and manage any complications.”
We have leveraged technology and developed clinical pathways to care for patients receiving CAR T-cell therapies. Our clinical teams have accomplished this by standardizing care across a network of several cell therapy programs.— Michael Tees, MD, director of Lymphoma Immune Effector Cell Therapy Program, Presbyterian/St. Luke’s Medical Center and Colorado Blood Cancer Institute in Denver, Colorado
The challenge and a solution
CAR T-cells can sometimes cause life-threatening inflammation once infused into patients. Most hospitals in the country typically admit and keep patients in the hospital for one to two weeks to monitor potential side effects. HCA Healthcare implements a 24/7 triage plan for early detection and management, which requires efficient communication between multiple teams to ensure proper care transition, medical assessment and inpatient observation/admission.
Recently, SCTCTN developed a remote patient monitoring care model to safely move 75% of its CAR T-cell therapy patients into an outpatient setting. This model reduced inpatient stays from an average of 16 days to about four days. It’s made possible through a partnership with Current Health, a home-care platform, using remote patient monitoring (RPM) technology and a nurse-staffed virtual clinical command center.
RPM includes a wearable device that continuously measures heart rate, temperature, respiration and oxygen saturation, and a tablet that allows the patient to interact with the virtual nurse team.

“Patients heal better outside the hospital if they can get these complicated therapies, which carry risk for significant complications, safely in the outpatient setting,” says Michael Tees, MD, director of Lymphoma Immune Effector Cell Therapy Program, Presbyterian/St. Luke’s Medical Center and Colorado Blood Cancer Institute in Denver, Colorado.
“We have leveraged technology and developed clinical pathways to care for patients receiving CAR T-cell therapies. Our clinical teams have accomplished this by standardizing care across a network of several cell therapy programs.”
The transition to providing this treatment in the outpatient setting has proven to be an enormous success and a game-changer for both hospital capacities and for patient access to the treatment.
“As we continue to innovate our care delivery models to enhance timely access, we are no longer bound by hospital bed capacity,” says Aravind Ramakrishnan, MD, medical director, Sarah Cannon Transplant & Cellular Therapy Program at St. David’s South Austin Medical Center in Austin, Texas.
HCA Healthcare is the only organization that has scaled up an RPM care model for CAR T-cell therapy across a multi-site network, and SCTCTN continues to innovate and refine the RPM care model through ongoing research and constant pursuit of industry-leading care.