Clinical labs shine in their crucial role during the pandemic.
When facing a problem as complex as COVID-19, accurate information is vital. Once the coronavirus took hold, HCA Healthcare’s clinical laboratories and partnering reference labs got busy—extremely busy—working around the clock to process tests as rapidly as possible to confirm infection. The goal: results in under 48 hours, prioritizing in-house patients and colleagues.
“We quickly began mobilizing efforts to get test results fast; it can’t be seven to 10 days,” says Dr. Heather Signorelli, DO, vice president and chief laboratory officer of the Clinical Services Group. “Initially, this was through short-term efforts with our reference labs, while we developed a long-term strategy with in-house testing. We relied heavily on our vendors, who were quickly trying to manage increasing demands for equipment and test kits.” Joanne Trout, CEO of Integrated Regional Laboratories in Fort Lauderdale, Fla., and the operational lead for our lab service line, was instrumental in managing these strategies, says Heather.
The organization continues to work with a variety of vendors on sharing test kits to ensure every facility has what they require. “We’re able to handle about 95% of our essential testing needs using the capacity we have today,” says Heather. “We can get results for every sample in less than 48 hours, and we can do a large proportion of that in-house or in our markets. That’s a huge change.”

“On the front lines, we’re caring for patients as if they are our own family members, and we’re dedicated to providing the most accurate and timely diagnostic information for our wonderful healthcare providers.”
—Matt Glynn
Lab manager
Alaska Regional Hospital
Anchorage, Alaska
We’re all in this together
In January and February—during the earliest stages of the pandemic—reference labs were completing between 10,000 and 20,000 tests daily. By late April, they were approaching 100,000. Heather explains one reason results could be available so quickly: unprecedented cooperation between HCA Healthcare and its vendors—and competitors.
“Stanford University let some of our hospitals in northern California send them tests before our automated platform was up and running,” says Heather. “To allow us testing for patient care is really great.”
The testing process also benefited from a laboratory restructuring across the enterprise that began rolling out in early 2019. “We had core teams of people who could address key laboratory issues, so they were ready,” says Heather. “They had already worked together and knew how to make things happen quickly.”
Within HCA Healthcare, there was streamlined cooperation. “We had multiple divisions—Gulf Coast and San Antonio, to name two—with excess capacity for testing,” says Heather. “They recognized that we had shortages, so they shipped equipment and test kits that were allocated specifically to them to other divisions that didn’t have anything in house, so we could have testing capabilities in other hotbed markets.”
While that was a short-term fix, says Heather, it allowed time to devise long-term strategies as facilities waited for additional vendors to make test kits available. “To see that level of cooperation between divisions—‘We know our sister facilities are struggling more than us, and we’re going to make sure that they’re taken care of’—was just amazing.”
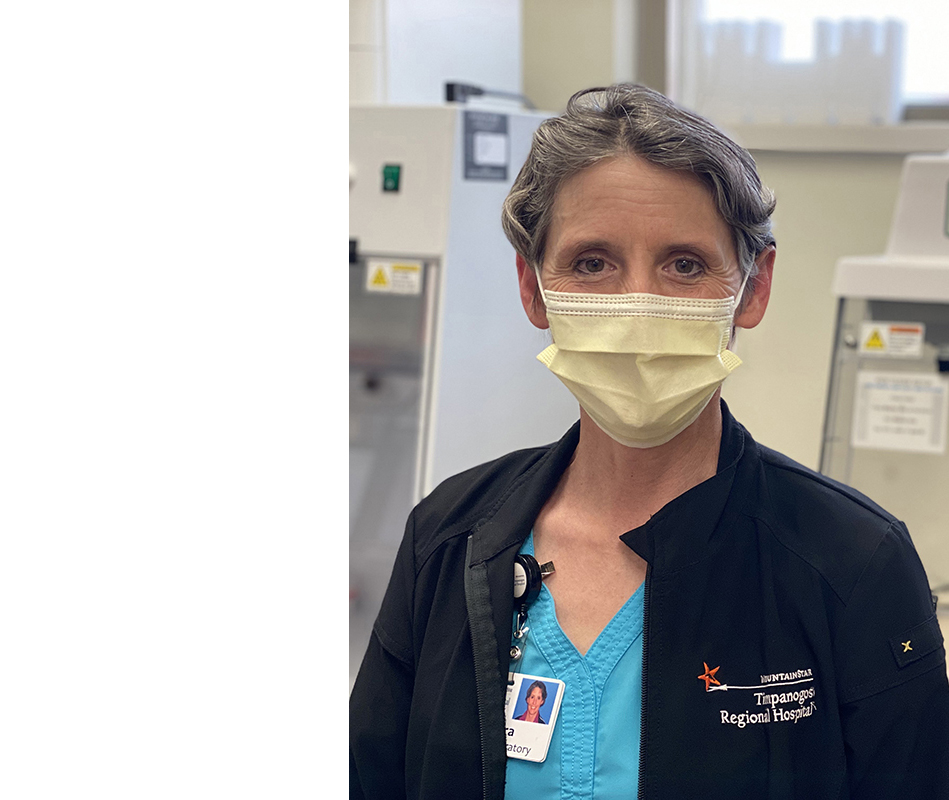
“The COVID-19 testing we’ve been involved in has made all the difference. We are trying to bring information and light to people who might be anxious and fearful about the situation around them.”
—Barbara Blanke
Lab director
Timpanogos
Regional Hospital
Orem, Utah
Applying lessons learned
After adapting to the initial surge in testing, Heather and Joanne continue to focus on capacity, constantly assessing how many tests are needed. “The next piece is, can we rely on the vendors providing testing capabilities to continue to have that level of manufacturing?” she says. “It’s making sure that one market isn’t relying solely on a single vendor because if that vendor becomes short on supplies, we’re in trouble. So we’re constantly trying to anticipate who’s our backup—and who’s our backup to the backup.”
Given the nature of the coronavirus, it will be an ongoing process requiring constant monitoring of the testing chain, Heather says. “Just as you solve one problem, you have another one to fix. It’s just part of the reality of trying to recover from this huge pandemic. We’re thinking about not only what our capacity looks like today, but what it will look like in July and September in case it does come back. We have to make sure we’re completely prepared for that as well.”


